Zika virus (ZIKV)
Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) in the genus Flavivirus and the family Flaviviridae. ZIKV was first isolated from a nonhuman primate in 1947 and from mosquitoes in 1948 in Africa, and ZIKV infections in humans were sporadic for half a century before emerging in the Pacific and the Americas. ZIKV is usually transmitted by the bite of infected mosquitoes. The clinical presentation of Zika fever is nonspecific and can be misdiagnosed as other infectious diseases, especially those due to arboviruses such as dengue and chikungunya.
ZIKV infection was associated with only mild illness prior to the large French Polynesian outbreak in 2013 and 2014, when severe neurological complications were reported, and the emergence in Brazil of a dramatic increase in severe congenital malformations (microcephaly) suspected to be associated with ZIKV.
Laboratory diagnosis of Zika fever relies on virus isolation or detection of ZIKV-specific RNA. Serological diagnosis is complicated by cross-reactivity among members of the Flavivirus genus. The adaptation of ZIKV to an urban cycle involving humans and domestic mosquito vectors in tropical areas where dengue is endemic suggests that the incidence of ZIKV infections may be underestimated. There is a high potential for ZIKV emergence in urban centers in the tropics that are infested with competent mosquito vectors such as Aedes aegypti and Aedes albopictus.
TRANSMISSION
MOSQUITO TRANSMISSION
In Africa, Zika virus exists in a transmission cycle involving nonhuman primates and forest-dwelling species of aedes mosquitoes (sylvatic transmission) Figure…In Asia, a sylvatic transmission cycle has not yet been identified. Several mosquito species, primarily belonging to the stegomyia and diceromyia subgenera of aedes, including A. africanus, A. luteocephalus, A. furcifer, and A. taylori, are likely enzootic vectors in Africa and Asia.
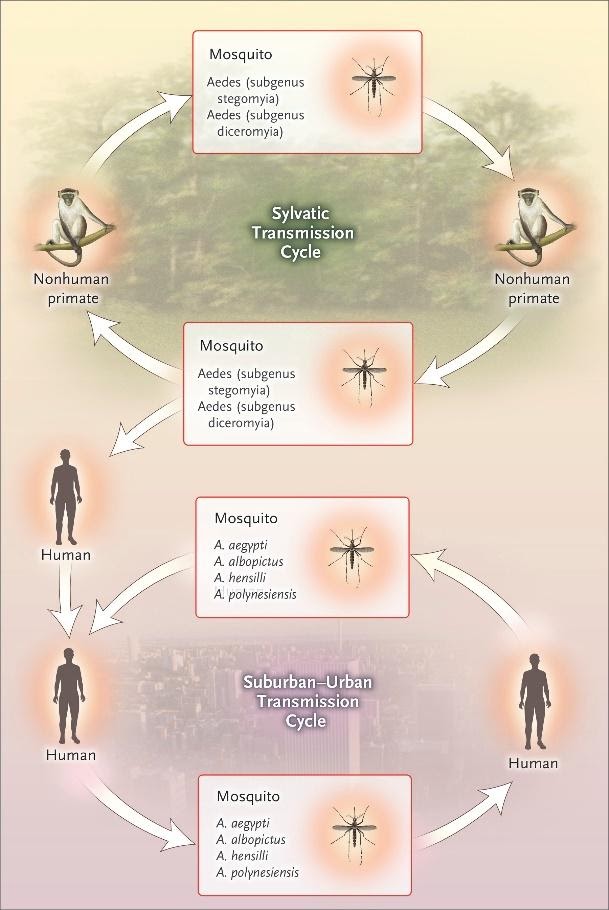
NONMOSQUITO TRANSMISSION
Substantial evidence now indicates that Zika virus can be transmitted from the mother to the fetus during pregnancy. Zika virus RNA has been identified in the amniotic fluid of mothers whose fetuses had cerebral abnormalities detected by ultrasonography and viral antigen and RNA have been identified in the brain tissue and placentas of children who were born with microcephaly and died soon after birth as well as in tissues from miscarriages. The frequency of and risk factors for transmission are unknown.
TREATMENT, PREVENTION, AND CONTROL
As with the other mosquito-borne flaviviruses, treatment for uncomplicated Zika virus infection focuses on symptoms. No Zika virus vaccine exists; thus, prevention and control measures center on avoiding mosquito bites, reducing sexual transmission, and controlling the mosquito vector.
Potentially effective methods of prevention that are focused on reducing infections among pregnant women include avoiding unnecessary travel to areas of ongoing Zika virus transmission, avoiding unprotected sexual contact with partners who are at risk for Zika virus infection, and using mosquito repellent, permethrin treatment for clothing bed nets window screens and air conditioning.
The most effective A. aegypti vector control relies on an integrated approach that involves elimination of A. aegypti mosquito breeding sites, application of larvicides, and application of insecticides to kill adult mosquitoes.
However, each of these approaches has substantial limitations. Communities are often mobilized to reduce A. aegypti breeding sites, but this strategy often fails, in part because of inconsistent participation among households and the presence of cryptic breeding sites in modern urban settings. Dengue control programs make extensive use of peri domestic insecticide spraying during outbreaks, but little evidence supports its efficacy as a single control intervention.
The application of larvicides and indoor residual spraying has been effective in some settings. Given these limitations, integrated prevention and vector-control approach combined with timely detection of illness, communication of up-to-date and correct information, and development of a rapid response that involves the community are recommended.
- Petersen, L.R., Jamieson, D.J., Powers, A.M. and Honein, M.A., 2016. Zika virus. New England Journal of Medicine, 374(16), pp.1552-1563.
- Musso, D. and Gubler, D.J., 2016. Zika virus. Clinical microbiology reviews, 29(3), pp.487-524.
